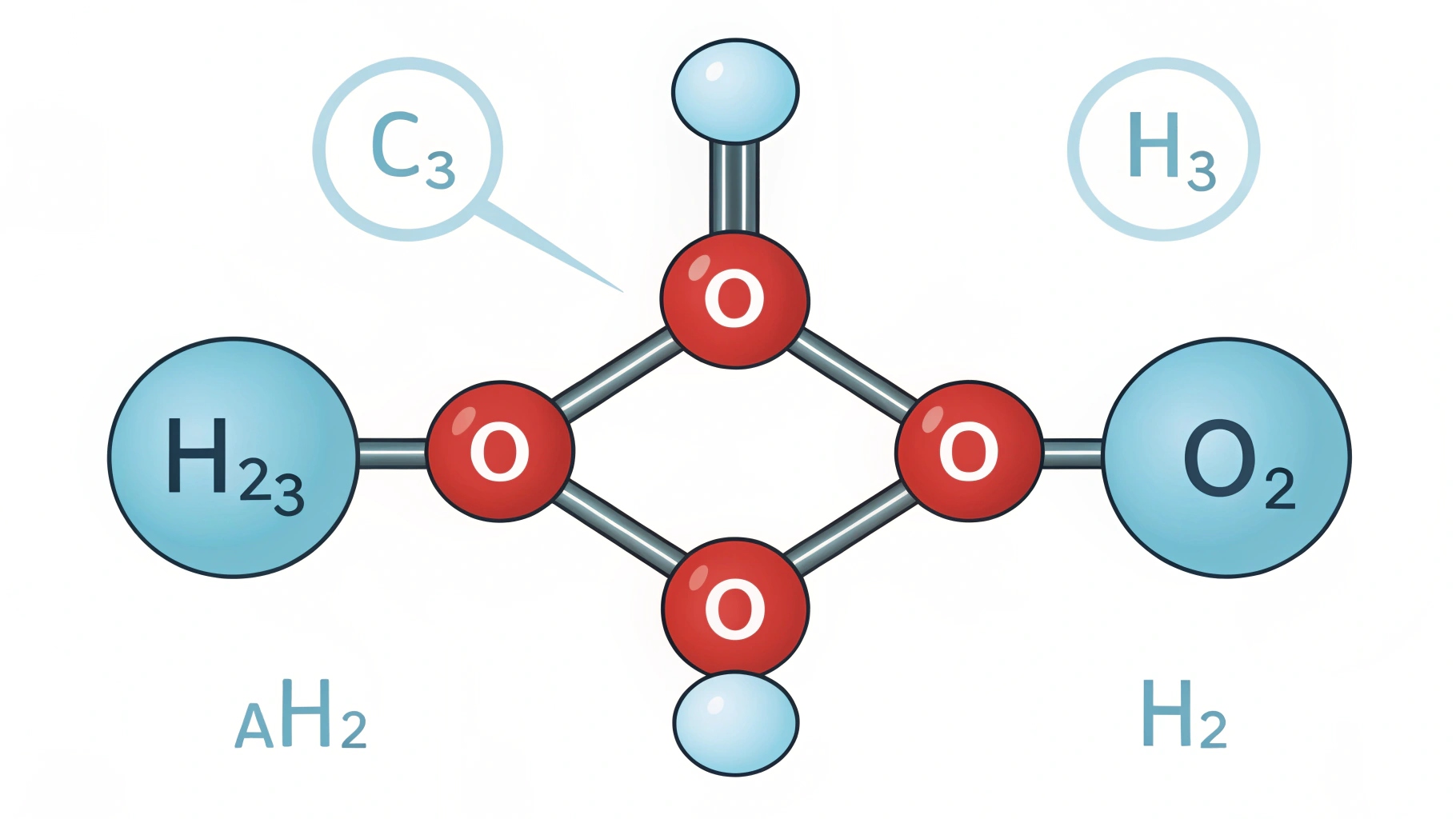
HCOOCH2H2O is an ester compound formed by the reaction of formic acid, methanol, and water. This molecule plays a significant role in various chemical and industrial processes, particularly in organic synthesis and solvent applications.
Known for its sweet, fruity odor, it shares properties with other esters and is used across multiple industries, including pharmaceuticals, coatings, and plastics. Its unique structure and reactivity make it valuable in both laboratory and industrial settings.
In this article, we will explore its chemical structure, properties, and diverse applications. Understanding HCOOCH2H2O reveals its importance in modern chemistry and industry.
Chemical Structure of HCOOCH2H2O
To break down the molecular formula HCOOCH2H2O, let’s dissect it into its constituent parts:
HCOO:
This portion represents a formate group (HCOO-), which is an ester of formic acid. Formic acid (HCOOH) is one of the simplest carboxylic acids. The formate group in this case is bonded with a methanol group.
CH2:
The next part of the molecule is a methylene group (CH2). Methanol (CH3OH) is a simple alcohol, and the CH2 here is attached to the ester group.
H2O:
Finally, the molecule contains a water group (H2O). Water is often involved in reactions that either produce or consume other substances, and here it plays a role in the overall structure.
The structure of HCOOCH2H2O is a formate ester that includes a water molecule. This type of ester is typically derived from a reaction between formic acid and methanol.
Properties of HCOOCH2H2O
HCOOCH2H2O shares many properties with other esters and alcohols, but it also has unique attributes due to the presence of both the ester and the water molecule. Some of its key properties include:
Physical State:
HCOOCH2H2O is typically a liquid at room temperature. It has a slightly sweet or fruity odor, similar to other ester compounds.
Solubility:
Being an ester, HCOOCH2H2O is soluble in polar solvents like water and alcohols. This solubility plays an important role in its applications in various chemical processes.
Boiling Point:
Esters like HCOOCH2H2O tend to have relatively low boiling points compared to other organic compounds, making them easy to evaporate and useful as solvents in various industrial processes.
Reactivity:
The ester group can undergo hydrolysis, breaking down into methanol and formic acid when exposed to water and heat. This reactivity is central to the compound’s role in industrial synthesis and in natural biochemical cycles.
Chemical Stability:
In the absence of water or acid catalysts, the ester bond in HCOOCH2H2O is relatively stable, but under the right conditions, it can easily break down to its constituent components.
Synthesis of HCOOCH2H2O
The most common method of synthesizing HCOOCH2H2O involves the reaction between methanol and formic acid. The esterification reaction proceeds as follows:
Formic acid (HCOOH) + Methanol (CH3OH) → HCOOCH3 + H2O
In the presence of a catalyst, such as sulfuric acid, this reaction typically leads to the formation of formate esters. Depending on the conditions of the reaction, water may be involved, leading to a modified version of this ester with additional H2O (water) molecules attached.
Applications of HCOOCH2H2O
HCOOCH2H2O has various uses in both industrial and laboratory settings. Some of the major applications of this compound include:
Solvent in Chemical Synthesis:
As a polar solvent, HCOOCH2H2O is used in chemical synthesis reactions, where it can dissolve both hydrophilic and hydrophobic substances. This makes it valuable in organic chemistry laboratories, particularly in reactions requiring mild solvents.
Intermediate in Pharmaceutical Production:
This compound can serve as an intermediate in the production of more complex pharmaceuticals. The formate ester structure is useful in synthesizing various drugs, especially in the modification of active pharmaceutical ingredients.
Biochemical Reactions:
In biological systems, esters like HCOOCH2H2O are involved in biochemical processes, such as enzyme catalysis, where they can serve as reactants or substrates in metabolic cycles.
Solvent in Paints and Coatings:
Like many other esters, HCOOCH2H2O can be utilized as a solvent in the formulation of paints, coatings, and varnishes. It helps dissolve pigments and other components, providing an even consistency.
Plasticizers and Additives:
Due to its ability to modify the properties of polymers, HCOOCH2H2O can act as a plasticizer in the manufacture of plastics. It reduces brittleness and enhances flexibility in certain plastic materials.
FAQs
1. What is HCOOCH2H2O?
HCOOCH2H2O is an ester compound formed from formic acid, methanol, and water, commonly used in chemical synthesis and industrial applications.
2. How is HCOOCH2H2O synthesized?
It is synthesized through an esterification reaction between methanol and formic acid, typically catalyzed by sulfuric acid.
3. Is HCOOCH2H2O toxic?
It is generally not highly toxic, but care should be taken to avoid prolonged exposure, especially through inhalation or skin contact.
4. What are the physical properties of HCOOCH2H2O?
It is a liquid at room temperature, soluble in water and alcohol, with a low boiling point and a sweet, fruity odor typical of esters.
5. Can HCOOCH2H2O be used as a solvent?
Yes, it is commonly used as a solvent in chemical synthesis, especially for dissolving organic and inorganic compounds.
6. What industries use HCOOCH2H2O?
Industries such as pharmaceuticals, coatings, plastics, and fragrance production utilize HCOOCH2H2O in various applications.
7. What happens when HCOOCH2H2O is heated?
When heated, HCOOCH2H2O can undergo hydrolysis, breaking down into methanol and formic acid, especially in the presence of water.
8. How does HCOOCH2H2O affect the environment?
If disposed of properly, HCOOCH2H2O does not present significant environmental hazards but should be treated before being released into nature.
9. What are the health risks of HCOOCH2H2O?
Prolonged exposure can irritate the skin, eyes, or respiratory system, requiring safety precautions when handling the chemical.
10. What is the role of HCOOCH2H2O in biological systems?
It acts as a substrate in biochemical reactions and can be involved in enzymatic processes within biological systems.
Conclusion
HCOOCH2H2O, though not a household name, plays a significant role in the fields of chemistry, industry, and even biology. As an ester, it possesses a variety of properties that make it suitable for use in chemical synthesis, pharmaceuticals, and industrial applications such as solvents and plasticizers.
By understanding its structure, properties, and applications, we can better appreciate the versatility of this compound and its importance in both scientific and industrial contexts.
Whether you encounter it in the laboratory, the pharmaceutical industry, or in industrial production, HCOOCH2H2O is an essential part of modern chemical science.














+ There are no comments
Add yours